The ultrasonic speeds,
u and viscosities,
η of the binary mixtures of methyl acrylate with benzene, toluene,
o-xylene,
m-xylene,
p-xylene, and mesitylene over the whole mole fraction range were measured at six different temperatures and at atmospheric pressure. From the experimental data, the excess isentropic compressibility,
KSE, excess ultrasonic speed,
uE, excess molar isentropic compressibility,
Ks,mE, excess specific impedance,
ZEand deviations in viscosity, Δ
ηhave been calculated. The partial molar isentropic compressions,
Ks,m,1 and
Ks,m,2, and excess partial molar isentropic compressions, ${\mathop K\limits^{ - E}}$
s,m,1 and ${\mathop K\limits^{ - E}}$
s,m,2 over the whole composition range, partial molar isentropic compressions,
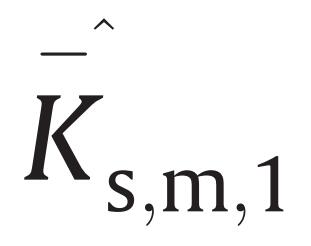
and
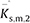
, and excess partial molar isentropic compressions, $\mathop K\limits^{ - \hat E} $
s,m,1 and $\mathop K\limits^{ - \hat E} $
s,m,2 of the components at infinite dilution have also been calculated. The results specified the existence of weak interactions between unlike molecules, and these interactions follow the order: benzene > toluene >
p-xylene >
m-xylene >
o-xylene > mesitylene. The magnitude of interactions was found to be dependent on the number and position of the methyl groups in these aromatic hydrocarbons.
 京公网安备 11010102001993号
京公网安备 11010102001993号 





 本期目录
本期目录